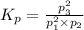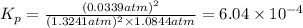Chemistry, 06.08.2019 05:10, alexsk6357
What is the value of kp for the reaction of nitrogen and oxygen to make dinitrogen monoxide if the equilibrium partial pressures of nitrogen is 1.3241 atm, the partial pressure of oxygen is 1.0844 atm and the partial pressure of dinitrogen monoxide is 0.0339 atm?

Answers: 3
Similar questions

Chemistry, 24.06.2019 18:00, LunaShiner
Answers: 1

Chemistry, 02.08.2019 23:30, montgomerykarloxc24x
Answers: 3

Chemistry, 14.09.2019 11:10, 1991987
Answers: 1
Do you know the correct answer?
What is the value of kp for the reaction of nitrogen and oxygen to make dinitrogen monoxide if the e...
Questions in other subjects:

Arts, 13.11.2020 18:50

SAT, 13.11.2020 18:50

Mathematics, 13.11.2020 18:50





Mathematics, 13.11.2020 18:50


Physics, 13.11.2020 18:50


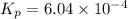
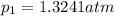
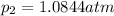
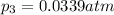
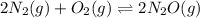
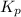 will be given as:
will be given as: