
When kcl dissolves in water
a) the cl- ions are attracted to dissolved k+ ions.
b) the cl- ions are attracted to the partially negative oxygen atoms of the water molecules.
c) the k+ions are attracted to cl- ions on the kcl crystal.
d) the k+ ions are attracted to the partially negative oxygen atoms of the water molecules.
e) the k+ions are attracted to the partially positive hydrogen atoms of the water molecules.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3



Chemistry, 23.06.2019 13:30, bossboybaker
What happens to acetone molecules when you add heat to a beaker of liquid acetone?
Answers: 1
Do you know the correct answer?
When kcl dissolves in water
a) the cl- ions are attracted to dissolved k+ ions.
b) the...
a) the cl- ions are attracted to dissolved k+ ions.
b) the...
Questions in other subjects:


Computers and Technology, 22.01.2020 04:31


Mathematics, 22.01.2020 04:31







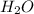 . And, due to difference in electronegativity of both hydrogen and oxygen atom there will be a partial positive charge on hydrogen atom and a partial negative charge on oxygen atom.
. And, due to difference in electronegativity of both hydrogen and oxygen atom there will be a partial positive charge on hydrogen atom and a partial negative charge on oxygen atom.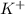 and
and 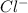 . As oxygen atom of water has partial positive charge so it will attract
. As oxygen atom of water has partial positive charge so it will attract 




