
Chemistry, 06.08.2019 04:20, kenzielema12
To a 25.00 ml volumetric flask, a lab technician adds a 0.150 g sample of a weak monoprotic acid, ha , and dilutes to the mark with distilled water. the technician then titrates this weak acid solution with 0.0969 m koh . she reaches the endpoint after adding 43.81 ml of the koh solution. determine the number of moles of the weak acid in the solution.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
Do you know the correct answer?
To a 25.00 ml volumetric flask, a lab technician adds a 0.150 g sample of a weak monoprotic acid, ha...
Questions in other subjects:


Mathematics, 06.04.2020 20:16





Mathematics, 06.04.2020 20:17

Social Studies, 06.04.2020 20:17

Mathematics, 06.04.2020 20:17

Mathematics, 06.04.2020 20:17


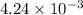 moles.
moles.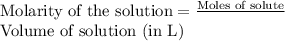
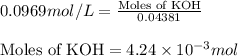
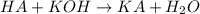
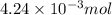 of KOH will react with =
of KOH will react with = 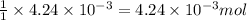 of weak monoprotic acid.
of weak monoprotic acid.



