
Chemistry, 06.08.2019 03:30, lindsaynielsen13
The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. if you were to react 93.3 g of potassium chlorate with excess red phosphorus (p4), to produce tetraphosphorus decaoxide and potassium chloride, what mass of tetraphosphorus decaoxide would be produed?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 06:40, Taylor73836
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1

Chemistry, 23.06.2019 07:00, Bassoonist
How does science use models to gain a better understanding of concepts?
Answers: 1

Chemistry, 23.06.2019 13:40, gtamods402
Which of the following volumes is the smallest? a) one microliter b)one deciliter d)one liter c)one milliliter
Answers: 2
Do you know the correct answer?
The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a...
Questions in other subjects:











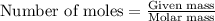 ....(1)
....(1)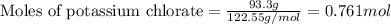
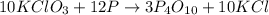
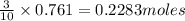 of tetraphosphorus decaoxide
of tetraphosphorus decaoxide




