
Solve for the standard entropy change (δs⁰) with each reaction below. as practice, try to predict what the sign would be before you solve it and see if it matches up.
a. n2(g) + 3 h2(g) ⇌ 2nh3(g)
b. nh4cl(s) ⇌ nh3(g) + hcl(g)
c. co(g) + 2h2(g) ⇌ ch3oh(l)
d. li3n(s) + 3h2o(l) ⇌ 3 lioh(aq) + nh3(g)

Answers: 3
Similar questions

Chemistry, 28.06.2019 06:30, luna163
Answers: 3


Chemistry, 13.07.2019 15:30, agyinbriana
Answers: 1
Do you know the correct answer?
Solve for the standard entropy change (δs⁰) with each reaction below. as practice, try to predict wh...
Questions in other subjects:


Chemistry, 31.07.2020 16:01





Social Studies, 31.07.2020 16:01

Physics, 31.07.2020 16:01



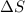 means change in entropy. As entropy means the measure of randomness. This means that more randomly molecules of a substance are moving more will be its entropy.
means change in entropy. As entropy means the measure of randomness. This means that more randomly molecules of a substance are moving more will be its entropy.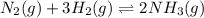 , total 4 moles of gases form 2 mole of a gas. This means there is decrease in number of moles. As a result, there will be decrease in entropy. So, sign of
, total 4 moles of gases form 2 mole of a gas. This means there is decrease in number of moles. As a result, there will be decrease in entropy. So, sign of 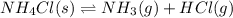 , total 1 mole of solid substance is forming total 2 moles of gases. That is, there is increase in entropy. So, sign of
, total 1 mole of solid substance is forming total 2 moles of gases. That is, there is increase in entropy. So, sign of 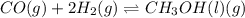 , total 2 moles of gases are forming total 1 mole of liquid methanol. That is, there is decrease in entropy. So, sign of
, total 2 moles of gases are forming total 1 mole of liquid methanol. That is, there is decrease in entropy. So, sign of 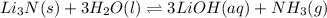 , total 2 moles of substance is forming total 4 moles of substance. That is, there is increase in entropy so, sign of
, total 2 moles of substance is forming total 4 moles of substance. That is, there is increase in entropy so, sign of 




