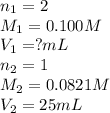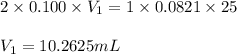Chemistry, 06.08.2019 01:30, heavendavis101
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(aq) + 2 h2o(l) calculate the volume of 0.100 m sulfuric acid required to neutralize 25.0 ml of 0.0821 m koh.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 23.06.2019 04:50, mia36492
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table. state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
Do you know the correct answer?
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(...
Questions in other subjects:


History, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01



Mathematics, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01



Mathematics, 23.10.2020 04:01


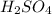 comes out to be 10.2625 mL.
comes out to be 10.2625 mL.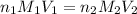
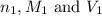 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 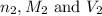 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.