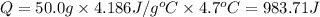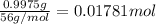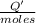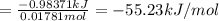Chemistry, 06.08.2019 01:20, larissacrystalow8g2w
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperature is 22.3 degrees celsius. after addition of the solid, the solution temperature is raised to about 27.0 degrees celsius. the substance is known to have a molar mass of about 56 g/mol. calculate the enthlapy of solution in kj/mol.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, mannster03
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Do you know the correct answer?
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperatur...
Questions in other subjects:


Mathematics, 15.12.2020 21:50

Chemistry, 15.12.2020 21:50



Health, 15.12.2020 21:50

Mathematics, 15.12.2020 21:50