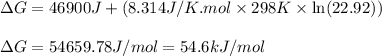Chemistry, 06.08.2019 01:10, candymorgan81
Consider the following reaction: co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at25 ∘c under these conditions: pco2pccl4pcocl2===0.140atm0.180atm0 .760atm δg∘f for co2(g) is −394.4kj/mol, δg∘f for ccl4(g) is −62.3kj/mol, and δg∘f for cocl2(g) is −204.9kj/mol. express the energy change in kilojoules per mole to one decimal place.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Do you know the correct answer?
Consider the following reaction: co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at25 ∘c un...
Questions in other subjects:









Biology, 25.07.2019 22:00

History, 25.07.2019 22:00


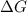 for the reaction is 54.6 kJ/mol
for the reaction is 54.6 kJ/mol 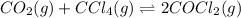
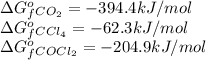
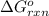 for the reaction, we use the equation:
for the reaction, we use the equation:![\Delta G^o_{rxn}=\sum [n\times \Delta G_f(product)]-\sum [n\times \Delta G_f(reactant)]](/tpl/images/0171/9686/1c133.png)
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(COCl_2)})]-[(1\times \Delta G^o_f_{(CO_2)})+(1\times \Delta G^o_f_{(CCl_4)})]](/tpl/images/0171/9686/08d2c.png)
![\Delta G^o_{rxn}=[(2\times (-204.9))-((1\times (-394.4))+(1\times (-62.3)))]\\\Delta G^o_{rxn}=46.9kJ=46900J](/tpl/images/0171/9686/b07a7.png)
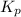 for the given reaction:
for the given reaction: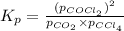
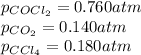
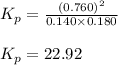
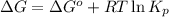
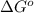 = Standard gibbs' free energy change of the reaction = 46900 J
= Standard gibbs' free energy change of the reaction = 46900 J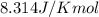
![25^oC=[25+273]K=298K](/tpl/images/0171/9686/df1f6.png)