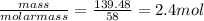Chemistry, 05.08.2019 22:30, izzynikkie
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in nail polish remover: c3h6o(l)+4 o2(g)→3 co2(g)+3 h2o(g)δh°rxn=−1790 kj if a bottle of nail polish remover contains 177 ml of acetone, how much heat is released by its complete combustion? the density of acetone is 0.788 g/ml.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2
Do you know the correct answer?
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in n...
Questions in other subjects:





English, 22.02.2021 19:30


Mathematics, 22.02.2021 19:30

Spanish, 22.02.2021 19:30

Mathematics, 22.02.2021 19:30

Mathematics, 22.02.2021 19:30