
Chemistry, 05.08.2019 22:20, weridness80
For the titration of a weak acid with a strong base what is the pka of the weak acid if the ph is 6.72 at the equilvalence point (100% t), 9.12 at the end point, and 4.23 at 50% t? answer with one sig fig.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2


Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
Do you know the correct answer?
For the titration of a weak acid with a strong base what is the pka of the weak acid if the ph is 6....
Questions in other subjects:









Physics, 09.11.2020 22:00


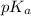 value.
value.![[A^{-}]](/tpl/images/0171/8766/fe74d.png)
![pK_{a} + log \frac{[A^{-}]}{[HA]}](/tpl/images/0171/8766/24c3e.png)
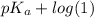 (as [HA] =
(as [HA] = 




