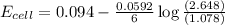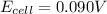Chemistry, 05.08.2019 22:20, baby092000
Consider the cell described below at 287 k: pb | pb2+ (1.07 m) || fe3+ (2.13 m) | fe given the standard reduction potentials found on the sheet attached to the exam, calculate the cell potential after the reaction has operated long enough for the [fe3+] to have changed by 1.052 m.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 01:30, Elliendc7939
List and describe the neurological effects of the vocs and other air pollutants, as described by dr. theo colborn
Answers: 2
Do you know the correct answer?
Consider the cell described below at 287 k: pb | pb2+ (1.07 m) || fe3+ (2.13 m) | fe given the stan...
Questions in other subjects:


History, 08.03.2021 03:30




Mathematics, 08.03.2021 03:30

English, 08.03.2021 03:30


Mathematics, 08.03.2021 03:30


![E^0_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0171/8681/82211.png)
![E^0_{[Fe^{3+}/Fe]}=-0.036V](/tpl/images/0171/8681/543db.png)
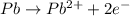
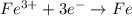
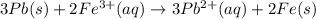
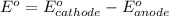
![E^o=E^o_{[Fe^{3+}/Fe]}-E^o_{[Pb^{2+}/Pb]}](/tpl/images/0171/8681/1c066.png)
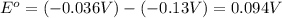
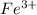 changed by 1.052 M.
changed by 1.052 M.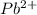 = 1.07 M
= 1.07 M![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Pb^{2+}]}{[Fe^{3+}]}](/tpl/images/0171/8681/72e91.png)
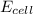 = emf of the cell = ?
= emf of the cell = ?