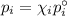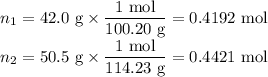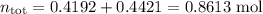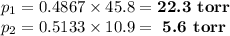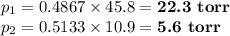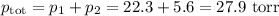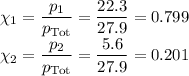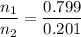Chemistry, 05.08.2019 21:20, DragonLovely
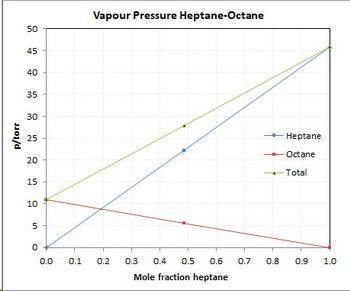
Asolution contains 42.0 g of heptane (c7h16) and 50.5 g of octane (c8h18) at 25 ∘c. the vapor pressures of pure heptane and pure octane at 25 ∘c are 45.8 torr and 10.9 torr, respectively. assuming ideal behavior, calculate each of the following. (note that the mole fraction of an individual gas component in an ideal gas mixture can be expressed in terms of the component's partial pressure.)a.) the vapor pressure of each of the solution components in the mixtureb.) the total pressure above the solutionc.) the composition of the vapor in mass percentd.) why is the composition of the vapor different from the composition of the solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, kat2177
The electron configurations of two different atoms are shown below. each yellow electron has a charge of 1−, and the net charge of each nucleus is shown. these atoms will combine with bond. a. an ionic b. a positive c. a negative d. a covalent plzzz mee with !
Answers: 1

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Do you know the correct answer?
Asolution contains 42.0 g of heptane (c7h16) and 50.5 g of octane (c8h18) at 25 ∘c. the vapor pressu...
Questions in other subjects:


Chemistry, 07.05.2021 21:50

Mathematics, 07.05.2021 21:50




History, 07.05.2021 21:50



Mathematics, 07.05.2021 21:50