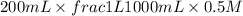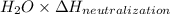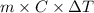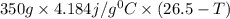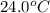Chemistry, 05.08.2019 19:10, camrenp9889
When 150. ml of 0.400 m h+ are mixed with 200. ml of 0.500 m oh-, the final temperature of the solution is 26.5°c. what was the initial temperature of the solution before the reaction occurred? assume that the solution has a total mass of 350. g and a specific heat capacity of 4.184 j/g°c. the enthalpy of neutralization for the reaction is -62.0 kj/mol of water produced.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2
Do you know the correct answer?
When 150. ml of 0.400 m h+ are mixed with 200. ml of 0.500 m oh-, the final temperature of the solut...
Questions in other subjects:

History, 30.12.2019 05:31





Mathematics, 30.12.2019 05:31

History, 30.12.2019 05:31


Computers and Technology, 30.12.2019 05:31


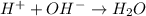
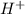 = volume × concentration of
= volume × concentration of 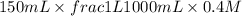
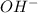 = volume × concentration of
= volume × concentration of