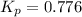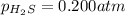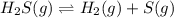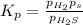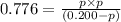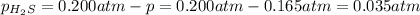Chemistry, 03.08.2019 03:30, kandikisses2101
At a certain temperature, the for the decomposition of h2s is 0.776. h2s(g)↽−−⇀h2(g)+s(g) initially, only h2s is present at a pressure of 0.200 atm in a closed container. what is the total pressure in the container at equilibrium?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
Do you know the correct answer?
At a certain temperature, the for the decomposition of h2s is 0.776. h2s(g)↽−−⇀h2(g)+s(g) initially...
Questions in other subjects:

Geography, 25.08.2019 12:50

World Languages, 25.08.2019 12:50





Physics, 25.08.2019 12:50

Mathematics, 25.08.2019 13:00