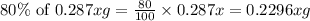Chemistry, 03.08.2019 00:30, trinigal83
The useful metal manganese can be extracted from the mineral rhodochrosite by a two-step in the first step, manganese(ii) carbonate and oxygen react to form manganese(iv) oxide and carbon dioxide: 2mnco3 + o2=2mno2 + 2co2in the second step, manganese(iv) oxide and aluminum react to form manganese and aluminum oxidide: 3mno2 + 4al = 3mn + 2al2o3 suppose the yield of the first step is 65.% and the yield of the second step is 80.%. calculate the mass of manganese(ii) carbonate required to make 8.0kg of manganese. be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kingteron5870
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1


Chemistry, 22.06.2019 19:50, nikoidurrant
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
Do you know the correct answer?
The useful metal manganese can be extracted from the mineral rhodochrosite by a two-step in the fir...
Questions in other subjects:

English, 24.06.2020 23:01


Biology, 24.06.2020 23:01


Mathematics, 24.06.2020 23:01


Mathematics, 24.06.2020 23:01

Business, 24.06.2020 23:01

History, 24.06.2020 23:01


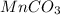 required are, 35 kg
required are, 35 kg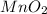 .
.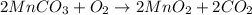
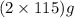 of
of 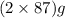 of
of 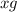 of
of 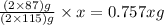 of
of 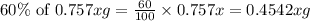
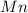 .
.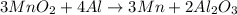
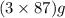 of
of 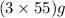 of
of 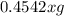 of
of 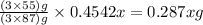 of
of