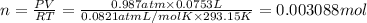Chemistry, 02.08.2019 22:20, shongmadi77
The reaction of solid aluminum with hydrochloric acid is used to make hydrogen gas in a laboratory experiment. the reaction is 2al(s) + 6hcl(aq) → 2alcl3(aq) + 3h2(g). the hydrogen gas is collected over water. how many moles of hydrogen gas were formed when 75.3 ml is collected at 20.0oc and 768.0 torr pressure? the vapor pressure of water at this temperature is 17.5 torr.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 23.06.2019 04:31, saladdressing1425
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
Do you know the correct answer?
The reaction of solid aluminum with hydrochloric acid is used to make hydrogen gas in a laboratory e...
Questions in other subjects:




Biology, 28.06.2019 00:00



Mathematics, 28.06.2019 00:00

Mathematics, 28.06.2019 00:00


Mathematics, 28.06.2019 00:00