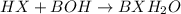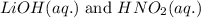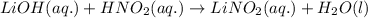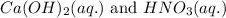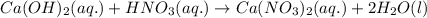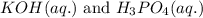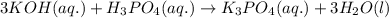Chemistry, 02.08.2019 22:20, Animallover100
Write the complete equation for the neutralization reactions that take place when the following water solutions are mixed. (if an acid has more than one acidic hydrogen, assume that there is enough base to remove all of them. assume that there is enough acid to neutralize all of the basic hydroxide ions.) a. lioh(aq) hno2(aq) b. co(oh)2(s) hno3(aq) c. h3po4(aq) koh(aq

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
Do you know the correct answer?
Write the complete equation for the neutralization reactions that take place when the following wate...
Questions in other subjects:





Biology, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01