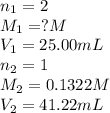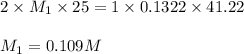Chemistry, 02.08.2019 21:40, arianabarber
A25.00-ml sample of an h2so4 solution of unknown concentration is titrated with a 0.1322 m koh solution. a volume of 41.22 ml of koh is required to reach the equivalence point. what is the concentration of the unknown h2so4 solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
Do you know the correct answer?
A25.00-ml sample of an h2so4 solution of unknown concentration is titrated with a 0.1322 m koh solut...
Questions in other subjects:




Computers and Technology, 01.07.2021 17:30




Mathematics, 01.07.2021 17:30



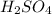 comes out to be 0.109 M.
comes out to be 0.109 M.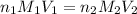
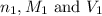 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 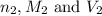 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.