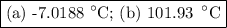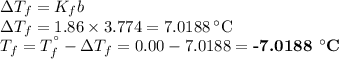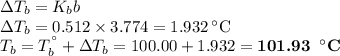Chemistry, 01.08.2019 05:10, pricillagarcia2002
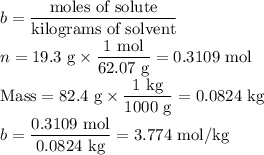
An ethylene glycol solution contains 19.3g of ethylene glycol (c2h6o2) in 82.4ml of water.
(a) compute the freezing point of the solution. (assume a density of 1.00 g/ml for water.)
(b)compute the boiling point of the solution. (assume a density of 1.00 g/ml for water.)
express your answer using five significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Do you know the correct answer?
An ethylene glycol solution contains 19.3g of ethylene glycol (c2h6o2) in 82.4ml of water.
(a)...
(a)...
Questions in other subjects:


Mathematics, 10.07.2019 09:10


Mathematics, 10.07.2019 09:10


Business, 10.07.2019 09:10

Mathematics, 10.07.2019 09:10

Mathematics, 10.07.2019 09:10


Mathematics, 10.07.2019 09:10