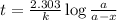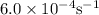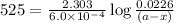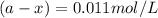Chemistry, 01.08.2019 05:10, jalenshayewilliams
Cyclopropane, c3h6, is converted to its isomer propylene, ch2=chch3, when heated. the rate law is first order in cyclopropane, and the rate constant is 6.0 × 10−4/s at 500°c. if the initial concentration of cy- clopropane is 0.0226 mol/l, what is the concentration after 525 s?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Do you know the correct answer?
Cyclopropane, c3h6, is converted to its isomer propylene, ch2=chch3, when heated. the rate law is fi...
Questions in other subjects:







Spanish, 03.05.2020 13:03

Mathematics, 03.05.2020 13:03

Social Studies, 03.05.2020 13:03

Mathematics, 03.05.2020 13:03


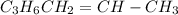
![Rate=k[C_3H_6]^1](/tpl/images/0157/0253/67ac9.png)