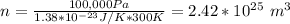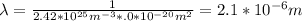Chemistry, 01.08.2019 04:30, ddatsman1730
What is the mean free path for the molecules in an ideal gas when the pressure is 100 kpa and the temperature is 300 k given that the collision cross-section for the molecules of that gas is 2.0 × 10-20 m2? boltzmann's constant is k = 1.38 × 10-23 j/k.

Answers: 1
Similar questions

Physics, 14.09.2019 09:30, mistermansour07
Answers: 1
Do you know the correct answer?
What is the mean free path for the molecules in an ideal gas when the pressure is 100 kpa and the te...
Questions in other subjects:


History, 27.09.2019 03:00

English, 27.09.2019 03:00


Geography, 27.09.2019 03:00

Mathematics, 27.09.2019 03:00

Health, 27.09.2019 03:00



Mathematics, 27.09.2019 03:00