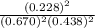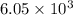Chemistry, 01.08.2019 03:20, makeithappen60
At 250°c an equilibrium mixture of sbcl3(g), cl2(g), and sbcl5(g) has the partial pressures 0.670 bar, 0.438 bar, and 0.228 bar, respectively. calculate the new equilibrium pressures if the volume of the reaction vessel is doubled.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 22:10, preachersgirl5
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
Do you know the correct answer?
At 250°c an equilibrium mixture of sbcl3(g), cl2(g), and sbcl5(g) has the partial pressures 0.670 ba...
Questions in other subjects:

Mathematics, 25.01.2021 21:00

English, 25.01.2021 21:00




Mathematics, 25.01.2021 21:00



Mathematics, 25.01.2021 21:00


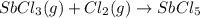
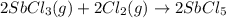
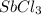 is 0.670 bar,
is 0.670 bar, 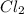 is 0.438 bar and
is 0.438 bar and 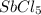 is 0.228 bar.
is 0.228 bar. ![K_{p} = \frac{[P_{SbCl_{5}}]^{2}}{[P_{SbCl_{3}}]^{2}[P_{Cl_{2}}]^{2}}](/tpl/images/0156/7126/bcfbb.png)