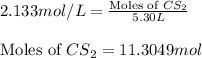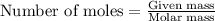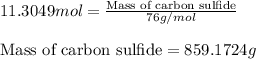Carbon disulfide is prepared by heating sulfur and charcoal. the chemical equation is s2(g)+c(s)↽−−⇀cs2(=9.40 at 900 k how many grams of cs2(g) can be prepared by heating 12.5 mol s2(g) with excess carbon in a 5.30 l reaction vessel held at 900 k until equilibrium is attained?

Answers: 2
Similar questions

Chemistry, 26.06.2019 12:20, mayaparness
Answers: 3

Chemistry, 24.07.2019 22:20, nikejose11
Answers: 2

Chemistry, 28.09.2019 16:00, KindaSmartPersonn
Answers: 1
Do you know the correct answer?
Carbon disulfide is prepared by heating sulfur and charcoal. the chemical equation is s2(g)+c(s)↽−−⇀...
Questions in other subjects:


Mathematics, 22.01.2021 16:40



English, 22.01.2021 16:40

Health, 22.01.2021 16:40


Mathematics, 22.01.2021 16:40



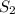 , we use the equation:
, we use the equation: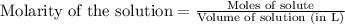 .....(1)
.....(1)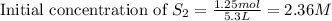
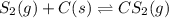
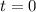 2.36
2.36 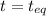 2.36 - x x
2.36 - x x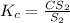

![[CS_2]=x](/tpl/images/0156/7037/d556c.png)
![[S_2]=2.36-x](/tpl/images/0156/7037/9691b.png)
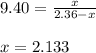
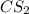 = 2.133 M
= 2.133 M