
Chemistry, 01.08.2019 03:10, dwilburn01
For a particular redox reaction, no is oxidized to no−3 and ag+ is reduced to ag . complete and balance the equation for this reaction in basic solution. the phases are optional. balanced reaction: no + ag^{+} -> no_{3}^{-} + ag no+ag+⟶no−3+ag

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, artemiscrock77041
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1


Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
Do you know the correct answer?
For a particular redox reaction, no is oxidized to no−3 and ag+ is reduced to ag . complete and bala...
Questions in other subjects:



Biology, 30.10.2020 22:50

Mathematics, 30.10.2020 22:50



Mathematics, 30.10.2020 22:50


History, 30.10.2020 22:50

Mathematics, 30.10.2020 22:50


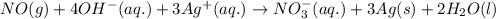
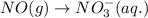
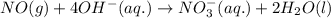
 ................(1)
................(1)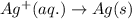
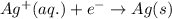 ...........(2)
...........(2)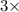 equation (2):
equation (2):



