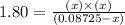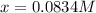Chemistry, 01.08.2019 02:20, tinapersaud1587
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80 at 250 ∘c a 0.349 mol sample of pcl5(g) is injected into an empty 4.00 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 2
Similar questions

Chemistry, 24.07.2019 23:10, amyeileen
Answers: 1

Chemistry, 16.09.2019 19:30, gracebuffum
Answers: 3

Chemistry, 02.10.2019 04:10, sssssaaaaaddddd5712
Answers: 1

Chemistry, 15.10.2019 18:20, thestarlexyp32wpj
Answers: 2
Do you know the correct answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80...
Questions in other subjects:

Mathematics, 01.03.2021 22:20

Mathematics, 01.03.2021 22:20


Health, 01.03.2021 22:20


Social Studies, 01.03.2021 22:20


Mathematics, 01.03.2021 22:20



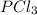 and
and 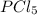 at equilibrium are, 0.0834 M and 0.00385 M
at equilibrium are, 0.0834 M and 0.00385 M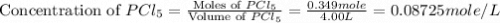
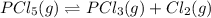
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0156/5529/73fe0.png)