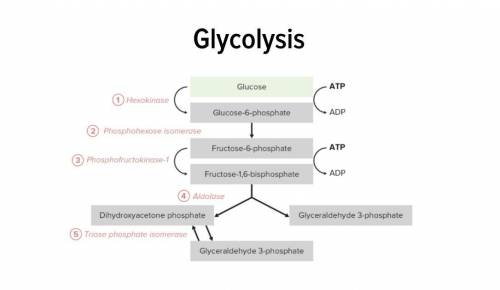
The free energy for the oxidation of glucose to co2 and water is -686 kcal/mol, and the free energy for the reduction of nad+ to nadh is +53 kcal/mol. why are only two molecules of nadh formed during glycolysis when it appears that as many as a dozen could be formed?

Answers: 3
Similar questions

Chemistry, 16.09.2019 23:30, andrespeerman
Answers: 1

Chemistry, 22.10.2019 19:00, cabieses23
Answers: 1
Do you know the correct answer?
The free energy for the oxidation of glucose to co2 and water is -686 kcal/mol, and the free energy...
Questions in other subjects:

Arts, 21.08.2019 10:30




Mathematics, 21.08.2019 10:30



Mathematics, 21.08.2019 10:30

Mathematics, 21.08.2019 10:30

Mathematics, 21.08.2019 10:30