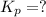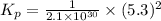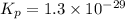Chemistry, 01.08.2019 01:20, christophergaudette0
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)kp=5.3 2no(g)⇌n2(g)+o2(g)kp=2.1×1030 use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: n2(g)+o2(g)+br2(g)⇌2nobr(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 01:30, Nakiahalogn4
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
Do you know the correct answer?
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)...
Questions in other subjects:


Social Studies, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00

Engineering, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00


Mathematics, 05.11.2020 01:00




 ;
; 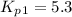
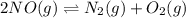 ;
; 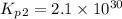
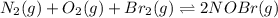 ;
;