
Chemistry, 01.08.2019 00:10, morris9878
The equilibrium constant for the dissociation of acetic acid at 25oc is 1.85x10-5. what will be the equilibrium concentration of h+ at 25oc, if the initial concentration of acetic acid was 0.100 m? ch3cooh (aq) ↔ ch3coo- (aq) + h+ (aq) give your answer to two significant figures and in decimal form.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 09:20, annapittbull12
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2
Do you know the correct answer?
The equilibrium constant for the dissociation of acetic acid at 25oc is 1.85x10-5. what will be the...
Questions in other subjects:

Mathematics, 18.01.2020 20:31

Physics, 18.01.2020 20:31

Geography, 18.01.2020 20:31



English, 18.01.2020 20:31

English, 18.01.2020 20:31

Mathematics, 18.01.2020 20:31



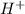 is
is 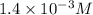
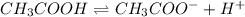
![\frac{[CH_{3}COO^{-}][H^{+}]}{[CH_{3}COOH]}](/tpl/images/0156/2235/23a00.png) =
= 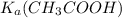
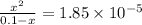
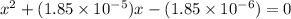
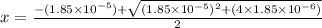 M
M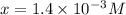
![[H^{+}]](/tpl/images/0156/2235/85507.png) =x=
=x=



