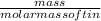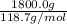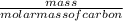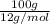Chemistry, 31.07.2019 23:30, gabischmid4340
Sno2 is reduced by carbon according to this reaction: sno2 + c sn + co2. how many liters of co2 are produced if 300.0 grams of tin are produced at stp? how many grams of sno2 are required to produce 1800.0 grams of tin? how much tin is produced per 100.0 grams of carbon used?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 05:30, jalynholden07
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 08:40, sugakookies1
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
Do you know the correct answer?
Sno2 is reduced by carbon according to this reaction: sno2 + c sn + co2. how many liters of co2 ar...
Questions in other subjects:

Chemistry, 07.06.2021 15:40






Mathematics, 07.06.2021 15:40


English, 07.06.2021 15:40

Physics, 07.06.2021 15:40


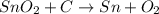
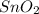 is [(mass of Sn) + (2 × mass of O)].
is [(mass of Sn) + (2 × mass of O)].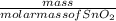
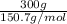
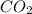 . Hence, 2.53 moles of
. Hence, 2.53 moles of