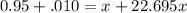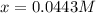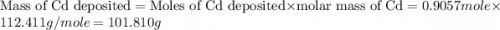Determine the mass of the metal at the cathode of the following galvanic cell when the cell is “dead” (at 298 k) assuming that the initial mass of both metal electrodes are 100 g and all solutions in the cell are exactly 1.0 l. fe | fe2+ (0.10 m) || cd2+ (0.95 m) | cd

Answers: 3
Similar questions

Physics, 05.08.2019 20:30, genyjoannerubiera
Answers: 3

Chemistry, 14.09.2019 08:30, joe805
Answers: 3

Chemistry, 14.09.2019 08:30, jwunder5859
Answers: 1
Do you know the correct answer?
Determine the mass of the metal at the cathode of the following galvanic cell when the cell is “dead...
Questions in other subjects:



Mathematics, 13.10.2020 14:01


Social Studies, 13.10.2020 14:01




History, 13.10.2020 14:01


![E^0_{[Fe^{2+}/Fe]}=-0.44V](/tpl/images/0156/0778/59478.png)
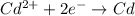
![E^0_{[Cd^{2+}/Cd]}=-0.40V](/tpl/images/0156/0778/d77be.png)
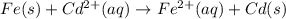
![E^0=E^0_{[Cd^{2+}/Cd]}-E^0_{[Fe^{2+}/Fe]}](/tpl/images/0156/0778/49ec3.png)
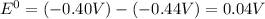
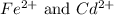 .
.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Fe^{2+}]}{[Cd^{2+}]}](/tpl/images/0156/0778/33164.png)
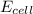 = emf of the cell = 0 (for dead cell)
= emf of the cell = 0 (for dead cell)![0=0.04-\frac{0.0592}{2}\log \frac{[Fe^{2+}]}{[Cd^{2+}]}](/tpl/images/0156/0778/3fae7.png)
![\frac{[Fe^{2+}]}{[Cd^{2+}]}=22.695](/tpl/images/0156/0778/c0251.png)
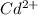 be, 'x'.
be, 'x'.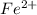 will be,
will be, ![22.695\times [Cd^{2+}]=22.695x](/tpl/images/0156/0778/44a3e.png)
![[Cd^{2+}]+[Fe^{2+}]=x+22.695x](/tpl/images/0156/0778/08dba.png)