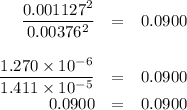Chemistry, 31.07.2019 22:20, Laners0219
The value of kc for the reaction between water vapor and dichlorine monoxide, h2o(g) 1 cl2o(g) 4 2 hocl(g) is 0.0900 at 25°c. determine the equilibrium concentrations of all three compounds at 25°c if the starting concentrations of both reactants are 0.00432 m and no hocl is present.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3


Chemistry, 23.06.2019 02:30, kieante01
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Do you know the correct answer?
The value of kc for the reaction between water vapor and dichlorine monoxide, h2o(g) 1 cl2o(g) 4 2 h...
Questions in other subjects:

SAT, 30.11.2021 01:50




SAT, 30.11.2021 01:50

Mathematics, 30.11.2021 01:50


Computers and Technology, 30.11.2021 01:50



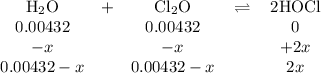
![K_{\text{c}} = \dfrac{\text{[HOCl]$^{2}$}}{\text{[H$_{2}$O][Cl$_2$O]}} = \dfrac{(2x)^{2}}{(0.00432 - x)^{2}} = 0.0900\\\\\begin{array}{rcl}\dfrac{4x^{2}}{(0.00432 - x)^{2}} &=& 0.0900\\ \dfrac{2x }{0.00432 - x} & = & 0.300\\2x & = & 0.300(0.00432 - x)\\2x & = & 0.001296 - 0.300x\\2.300x & = & 0.001296\\x & = & \mathbf{5.63\times 10^{-4}}\\\end{array}](/tpl/images/0155/9177/30633.png)