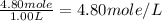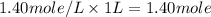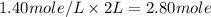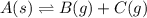Chemistry, 31.07.2019 21:20, adaakbulut9
A4.80 mol sample of solid a was placed in a sealed 1.00 l container and allowed to decompose into gaseous b and c. the concentration of b steadily increased until it reaches 1.40 m, where it remained constant. a(s)↽−−⇀b(g)+c(g) then, the container volume was doubled and equilibrium was re‑established. how many moles of a remain

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
Do you know the correct answer?
A4.80 mol sample of solid a was placed in a sealed 1.00 l container and allowed to decompose into ga...
Questions in other subjects:




Mathematics, 25.02.2021 19:20