
Chemistry, 31.07.2019 21:20, smelcher3900
Hydrogen peroxide decomposes into water and oxygen in a first-order process. h2o2(aq) --> h2o(l) + 1/2 o2(g)at 20.0 °c, the half-life for the reaction is 3.92 x 104 seconds. if the initial concentration of hydrogen peroxide is 0.52 m, what is the concentration after 7.00 days? 1.2 x 10-5 m0.034 m0.074 m0.22 m0.52 m

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1


Chemistry, 23.06.2019 01:30, elijahbebeastin
What are several ways to reduce the effect of friction
Answers: 2

Do you know the correct answer?
Hydrogen peroxide decomposes into water and oxygen in a first-order process. h2o2(aq) --> h2o(l)...
Questions in other subjects:

English, 21.04.2021 01:10


Mathematics, 21.04.2021 01:10


Mathematics, 21.04.2021 01:10

Mathematics, 21.04.2021 01:10



History, 21.04.2021 01:10

Biology, 21.04.2021 01:10


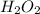 after 7.00 days is
after 7.00 days is 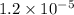 M
M![[H_{2}O_{2}]=[H_{2}O_{2}]_{0}\times (0.5)^{(\frac{t}{t_{0.5}})}](/tpl/images/0155/7555/57b7f.png)
![[H_{2}O_{2}]_{0}](/tpl/images/0155/7555/0e2b6.png) is initial concentration of
is initial concentration of 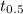 is half-life
is half-life![[H_{2}O_{2}]= 0.52\times (0.5)^{\frac{604800}{39200}}](/tpl/images/0155/7555/78886.png)
![[H_{2}O_{2}]](/tpl/images/0155/7555/955b6.png) =
= 



