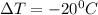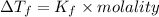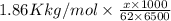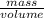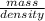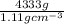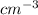Chemistry, 31.07.2019 20:30, shelbylynn17
Acommon antifreeze for car radiators is ethylene glycol, ch2(oh )ch2(oh ). how many millilite~s of this substance would you add to 6.5 l of water 1n the radiator if the coldest day in winter is - 20°c? would you keep this substance in the radiator in the summer to prevent the water from boiling? (the density and boiling point of ethylene glycol are 1.11 g cm^-3 and 470 k repsectively.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
Do you know the correct answer?
Acommon antifreeze for car radiators is ethylene glycol, ch2(oh )ch2(oh ). how many millilite~s of t...
Questions in other subjects:




English, 11.02.2020 05:54


History, 11.02.2020 05:54

Biology, 11.02.2020 05:54



Mathematics, 11.02.2020 05:55


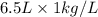 = 6.5 kg
= 6.5 kg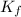 of water is 1.86 K kg/mol. And,
of water is 1.86 K kg/mol. And,