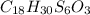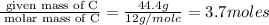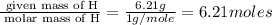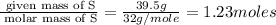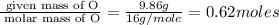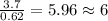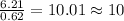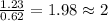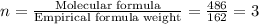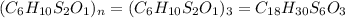Chemistry, 31.07.2019 20:30, live4dramaoy0yf9
6. allicin is the compound responsible for the characteristic smell of garlic. an analysis of the compound gives the following percent composition by mass: c: 44.4 percent; h: 6.21 percent; s: 39.5 percent; of: 9.86 percent. calculate its empirical formula. what is its molecular formula given that its molar mass is about 486g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1
Do you know the correct answer?
6. allicin is the compound responsible for the characteristic smell of garlic. an analysis of the co...
Questions in other subjects:



History, 21.02.2020 19:40



History, 21.02.2020 19:40



Mathematics, 21.02.2020 19:40


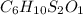 and the molecular of the compound is,
and the molecular of the compound is,