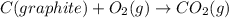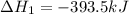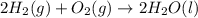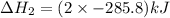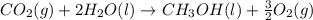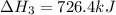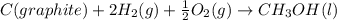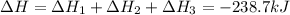Calculate δh for the reaction: c(graphite) + 2h 2(g) + 1/2 o 2(g) => ch 3oh(l) using the following information: c(graphite) + o 2 => co 2(g) δh o = -393.5 kj h 2(g) + 1/2 o 2 => h 2o(l) δh o = -285.8 kj ch 3oh (l) + 3/2 o 2(g) => co 2(g) + 2h 2o(l) δh o = -726.4 kj a. +238.7 kj b. -238.7 kj c. +548.3 kj d. -548.3 kj e. +904.5 kj

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1


Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
Do you know the correct answer?
Calculate δh for the reaction: c(graphite) + 2h 2(g) + 1/2 o 2(g) => ch 3oh(l) using the follow...
Questions in other subjects:


English, 25.09.2021 21:50


Mathematics, 25.09.2021 21:50

Business, 25.09.2021 21:50

Mathematics, 25.09.2021 21:50

Mathematics, 25.09.2021 21:50




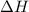 for the given reaction is -238.7 kJ
for the given reaction is -238.7 kJ