
Chemistry, 31.07.2019 19:10, brianlykid3042
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 ⋅ 10-4. calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 10-4. 0.859 0.0180 3.79 2.25 ⋅ 10-5 6.94

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
Do you know the correct answer?
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid...
Questions in other subjects:

History, 12.11.2020 05:20





World Languages, 12.11.2020 05:20


Mathematics, 12.11.2020 05:20

Biology, 12.11.2020 05:20

Mathematics, 12.11.2020 05:20


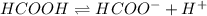
![\frac{[HCOO^{-}][H^{+}]}{[HCOOH]}=K_{a}(HCOOCH)](/tpl/images/0155/4252/45db7.png)
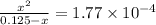
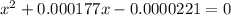
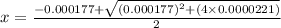
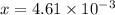 M
M![[H^{+}]=4.61\times 10^{-3}M](/tpl/images/0155/4252/38c16.png)
![\frac{[H^{+}]}{initial concentration of HCOOH}\times 100](/tpl/images/0155/4252/17262.png) =
= 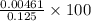 = 3.69%
= 3.69%



