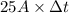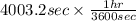Chemistry, 31.07.2019 18:20, chanel2371
Chromium plating can be applied by electrolysis to objects according to the following unbalanced half-reaction: cr2o72- + e- + h+→ cr(s) + h2ohow long (in hours) would it take to apply a chromium plating 0.010 mm thick to a car bumper with a surface area of 0.25 m2 in a cell with a current of 25.0 a? the density of chromium is 7.19 g/cm3.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
Do you know the correct answer?
Chromium plating can be applied by electrolysis to objects according to the following unbalanced hal...
Questions in other subjects:


Mathematics, 01.04.2021 22:00






Mathematics, 01.04.2021 22:00



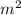 , current (I) = 25 A
, current (I) = 25 A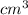
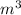 =
= 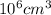
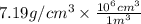
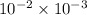 =
= 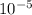 m.
m.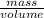
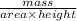
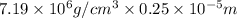
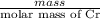
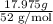
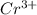 is 579000 C
is 579000 C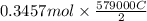 = 100080 C.
= 100080 C. 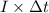
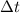 = time in seconds
= time in seconds