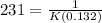Aparticular reactant decomposes with a half‑life of 109 s when its initial concentration is 0.280 m. the same reactant decomposes with a half‑life of 231 s when its initial concentration is 0.132 m.
1. determine the reaction order.
(a)1
(b)2
(c)0
2. what is the value and units of the rate constant for this reaction? =

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, KieraKimball
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 23.06.2019 04:31, mdarter
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

Chemistry, 23.06.2019 05:30, xarianna2007
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
Do you know the correct answer?
Aparticular reactant decomposes with a half‑life of 109 s when its initial concentration is 0.280 m....
Questions in other subjects:

Mathematics, 01.12.2020 07:10



Mathematics, 01.12.2020 07:10


Advanced Placement (AP), 01.12.2020 07:10

Mathematics, 01.12.2020 07:10

Mathematics, 01.12.2020 07:10


English, 01.12.2020 07:10


![\frac{1}{k[A_{0}]^{(n-1)}}](/tpl/images/0155/1180/be91f.png)
![K\frac{1}{[A_{0}]^{(n-1)} }](/tpl/images/0155/1180/1baec.png)
![\frac{(halflife_{1})}{(halflife_{2})}=\frac{[A_{2}]^{(n-1)}}{[A_{1}]^{(n-1)} }](/tpl/images/0155/1180/93500.png)
![\frac{109}{231}=\frac{[0.132]^{(n-1)}}{[0.280]^{(n-1)}}](/tpl/images/0155/1180/d6c94.png)
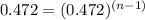
![halflife=\frac{1}{k[A_{0}]}](/tpl/images/0155/1180/b0fd9.png)