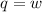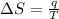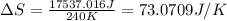Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume v1 to volume v2 = 9v1 at temperature t = 240 k. find (a) the work done by the gas and (b) the entropy change of the gas. (c) if the expansion is reversible and adiabatic instead of isothermal, what is the entropy change of the gas?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
Do you know the correct answer?
Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume v1 to volum...
Questions in other subjects:

Physics, 01.10.2019 01:00

Biology, 01.10.2019 01:00

Geography, 01.10.2019 01:00


Biology, 01.10.2019 01:00



English, 01.10.2019 01:00


English, 01.10.2019 01:00


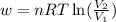
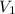 = initial volume of gas =
= initial volume of gas = 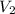 = final volume of gas =
= final volume of gas = 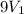
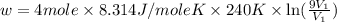
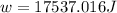
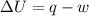
 = internal energy
= internal energy