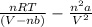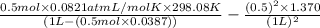Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3
Do you know the correct answer?
Calculate the pressure exerted by 0.5000 mol of n2 in a 1.-l container at 25.08c a. using the ideal...
Questions in other subjects:


Social Studies, 18.12.2019 05:31

Mathematics, 18.12.2019 05:31


History, 18.12.2019 05:31


Mathematics, 18.12.2019 05:31



Mathematics, 18.12.2019 05:31


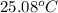 . Or in kelvin temperature will be (25.08 + 273) K = 298.08 K.
. Or in kelvin temperature will be (25.08 + 273) K = 298.08 K.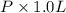 = 0.5 mol \times 0.0821 L atm/mol K \times 298.08 K[/tex]
= 0.5 mol \times 0.0821 L atm/mol K \times 298.08 K[/tex]![[P + a (\frac{n}{V})^{2}] (\frac{V}{n} - b)](/tpl/images/0149/5099/3685f.png) = RT
= RT