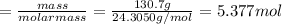Chemistry, 30.07.2019 04:10, luvpeaceandsocc6312
Assuming an efficiency of 49.50%, calculate the actual yield of magnesium nitrate formed from 130.7 g of magnesium and excess copper(ii) nitrate. mg+cu(no3)2⟶mg(no3)2+cu actual yield:

Answers: 2
Similar questions


Chemistry, 28.08.2019 07:30, ms0579930
Answers: 1

Chemistry, 17.09.2019 22:10, xbeatdroperzx
Answers: 1

Chemistry, 15.10.2019 04:10, badpotterchris
Answers: 1
Do you know the correct answer?
Assuming an efficiency of 49.50%, calculate the actual yield of magnesium nitrate formed from 130.7...
Questions in other subjects:


Arts, 17.11.2020 21:40



Chemistry, 17.11.2020 21:40

Mathematics, 17.11.2020 21:40

History, 17.11.2020 21:40



Health, 17.11.2020 21:40