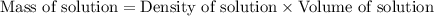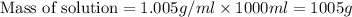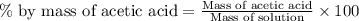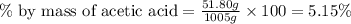Chemistry, 30.07.2019 02:20, jaureguilol1
What is the percent-by-mass concentration of acetic acid (ch3cooh) in a vinegar solution that contains 51.80 g acetic acid in a 1.000−l solution? the density of this solution is 1.005 g/ml.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 23:00, ashyding4800
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 04:31, 1Angel2Got3Brains
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1

Chemistry, 23.06.2019 10:00, Sariyahhall1
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
Do you know the correct answer?
What is the percent-by-mass concentration of acetic acid (ch3cooh) in a vinegar solution that contai...
Questions in other subjects:


Mathematics, 26.03.2021 05:00


Mathematics, 26.03.2021 05:00

Mathematics, 26.03.2021 05:00


History, 26.03.2021 05:00

Mathematics, 26.03.2021 05:00

Business, 26.03.2021 05:00

Social Studies, 26.03.2021 05:00