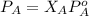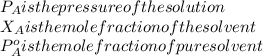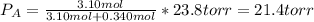Chemistry, 30.07.2019 02:10, keviongardner
If 0.340 mol of a nonvolatile nonelectrolyte are dissolved in 3.10 mol of water, what is the vapor pressure ph2o of the resulting solution? the vapor pressure of pure water is 23.8 torr at 25 ∘c . express your answer with the appropriate units.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
Do you know the correct answer?
If 0.340 mol of a nonvolatile nonelectrolyte are dissolved in 3.10 mol of water, what is the vapor p...
Questions in other subjects:



Biology, 24.06.2019 21:10

Mathematics, 24.06.2019 21:10

Mathematics, 24.06.2019 21:10

English, 24.06.2019 21:10

Mathematics, 24.06.2019 21:10