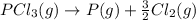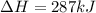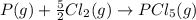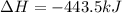Chemistry, 30.07.2019 01:20, jones03riley
Given the enthalpies of reaction: 2p(g) + 3cl2(g) → 2pcl3(g) dh = –574 kj 2p(g) + 5cl2(g) → 2pcl5(g) dh = –887 kj what is the enthalpy change of the following reaction: pcl3(g) + cl2(g) → pcl5(g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
Do you know the correct answer?
Given the enthalpies of reaction: 2p(g) + 3cl2(g) → 2pcl3(g) dh = –574 kj 2p(g) + 5cl2(g) → 2pcl5(g...
Questions in other subjects:

English, 26.10.2019 13:43


History, 26.10.2019 13:43



Health, 26.10.2019 13:43

History, 26.10.2019 13:43



Chemistry, 26.10.2019 13:43


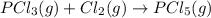
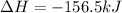
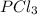 on reactant side. When we reversed an equation then the sign of enthalpy change is also changed.
on reactant side. When we reversed an equation then the sign of enthalpy change is also changed.