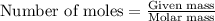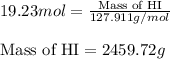Chemistry, 30.07.2019 01:10, justinbailey96
The standard enthalpy of formation, δh°f, of hi(g) is +26 kj mol-1. which of the following is the approximate mass of hi(g) that must decompose into h2(g) and i2(s) to release 500. kj of energy?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, kingsqueen883
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3



Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
Do you know the correct answer?
The standard enthalpy of formation, δh°f, of hi(g) is +26 kj mol-1. which of the following is the ap...
Questions in other subjects:

Advanced Placement (AP), 08.12.2020 05:30

Mathematics, 08.12.2020 05:30

Mathematics, 08.12.2020 05:30


Computers and Technology, 08.12.2020 05:30



English, 08.12.2020 05:30

Mathematics, 08.12.2020 05:30


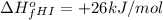
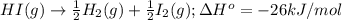
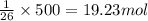 of hydrogen iodide will be decomposed.
of hydrogen iodide will be decomposed.