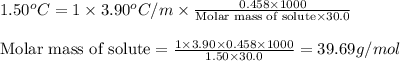Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, Britny2386
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1


Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
Do you know the correct answer?
Acompound weighing 0.458 g is dissolved in 30.0 g of acetic acid. the freezing point of the solution...
Questions in other subjects:


Mathematics, 28.09.2020 05:01

Mathematics, 28.09.2020 05:01

Mathematics, 28.09.2020 05:01


History, 28.09.2020 05:01

English, 28.09.2020 05:01




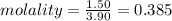
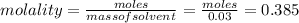
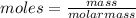
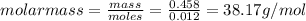
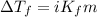
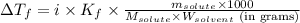
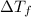 = Depression in freezing point = 1.50 K = 1.50°C (Change remains constant)
= Depression in freezing point = 1.50 K = 1.50°C (Change remains constant)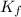 = molal freezing point elevation constant = 3.90°C/m
= molal freezing point elevation constant = 3.90°C/m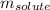 = Given mass of solute = 0.458 g
= Given mass of solute = 0.458 g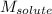 = Molar mass of solute (glucose) = ? g/mol
= Molar mass of solute (glucose) = ? g/mol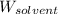 = Mass of solvent (acetic acid) = 30.0 g
= Mass of solvent (acetic acid) = 30.0 g