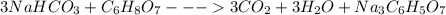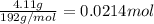Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water solution to produce a fizz as follows: 3nahco3 + c6h8o7 → 3co2 + 3h2o + na3c6h5o7 if 4.11 g of the citric acid (c6h8o7, mw = 192 g/mol) react with excess sodium bicarbonate (nahco3), how many grams of carbon dioxide (co2, mw = 44 g/mol) are formed as the solution fizzes?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, smhrosepetals
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
Do you know the correct answer?
Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water sol...
Questions in other subjects:


Mathematics, 11.03.2021 22:40

Mathematics, 11.03.2021 22:40




Mathematics, 11.03.2021 22:40


Mathematics, 11.03.2021 22:40

Social Studies, 11.03.2021 22:40