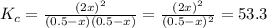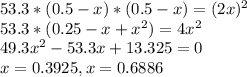Chemistry, 29.07.2019 21:10, sreeranjanig
At a certain temperature, the equilibrium constant, , for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(=53.3 at this temperature, 0.500 mol h2 and 0.500 mol i2 were placed in a 1.00 l container to react. what concentration of hi is present at equilibrium?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Falconpride4079
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
Do you know the correct answer?
At a certain temperature, the equilibrium constant, , for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(...
Questions in other subjects:






Mathematics, 14.12.2021 01:00

Chemistry, 14.12.2021 01:00

History, 14.12.2021 01:00



![K_{c}=\frac{[HI]^{2}}{[I_{2}][H_{2}]}](/tpl/images/0148/0680/ff3a2.png)
![[HI]={[HI]}_{0}+2x\\{[H_{2}]}={[H_{2}]}_{0}-x\\{[I_{2}]}={[I_{2}]}_{0}-x\\](/tpl/images/0148/0680/e5b4e.png)