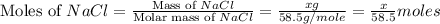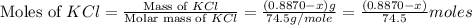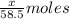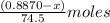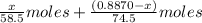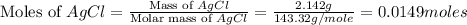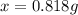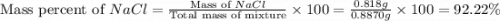Chemistry, 29.07.2019 20:20, nicolascorrea0207
A0.8870 g sample of a mixture of nacl and kcl is dissolved in water, and the solution is then treated with an excess of agno3 to yield 2.142 g of agcl. calculate the percent by mass of each compound in the mixture

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
Do you know the correct answer?
A0.8870 g sample of a mixture of nacl and kcl is dissolved in water, and the solution is then treate...
Questions in other subjects:

Geography, 09.06.2020 08:57

French, 09.06.2020 08:57


Mathematics, 09.06.2020 08:57


Geography, 09.06.2020 08:57



Mathematics, 09.06.2020 08:57


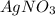 then the silver ion react with the chloride ion in both NaCl and KCl to form silver chloride.
then the silver ion react with the chloride ion in both NaCl and KCl to form silver chloride.